[試題] 106上 佘瑞琳 普通化學丙 第二次期中考
作者: oopzzozzo (π) 2017-11-27 22:58:40
課程名稱︰普通化學丙
課程性質︰必修
課程教師︰佘瑞琳
開課學院:生命科學院
開課系所︰生科系/生技系
考試日期(年月日)︰2017.11.27
考試時限(分鐘):100+10(延長)
試題 :
C = 3.00 ×10^8 m/s; h = 6.626 ×10^-34 J-s; R_H = 1.096776 ×10^7 m^-1; 1 eV = 1.6 ×10^-19 J
Gas constant: R = 8.314 J/mol-K=0.082 L-atm/mol-K; 1 L-atm = 101.3 J
I. Multiple choices questions(30%)
1. When 1 mol of a fuel is burned at constant pressure. It release 3453 kJ of heat (q) and does 12kJ of work (w) on surroundings. Which one of the followings is true?(△E: internal energy)
(a) q = -3453 kJ (b) w = -12 kJ (c) △E_system = 0 kJ (d) △H = -3453
2. For the following types of electromagnetic radiation: γ-ray, ultraviolet (UV), visible, and infrared(IR), which of the following is correct?
(a) Highest energy: γ-ray (b) Longest wavelength: IR
(c) Greatest frequency: UV (d) Lowext energy: visible
3. What is the full range of possible values of l for n = 3?
(a) 0 (b) 0 and 1 (c) 0, 1, and 2 (d) 0, 1, 2, and 3
4. These sets of quantum numbers are each supposed to specify an orbital. Choose the one that is erroneous.
(a)(3, 0, 0) (b)(2, 1, -1) (c)(1, 0, 0) (d)(4, 1, -2)
5. Which electron transition emits light of the highest frequency in the hydrogen atom?
(a) n_i=4 →n_f=3 (b) n_i=5 →n_f=4 (c) n_i=2 →n_f=5 (d) n_i=4 →n_f=2
6. Which of the following orbital diagram is correct for the ground-state electron configurations?
(a) [He]↑↑ ↑ ↑ ↑ (b) [He]↑↓ ↓↑ ↓ ↓
﹉﹉2s ﹉﹉ ﹉﹉ ﹉﹉2p ﹉﹉2s ﹉﹉ ﹉﹉ ﹉﹉2p
(c) [He]↑ ↓↑ ↓↑ ↓↑ (d) [He]↑↓ ↑ ↓ ↑
﹉﹉2s ﹉﹉ ﹉﹉ ﹉﹉2p ﹉﹉2s ﹉﹉ ﹉﹉ ﹉﹉2p
7. How many orbitals have the quantum values of n = 3 and l = 2?
(a) 3 (b) 5 (c) 7 (d) 9 (e) 10 (f) 14
8. Choose the valence shell electron configurations of the alkali metals.
(a) ns^1 (b) ns^2 (c)ns^2np1 (d) ns^2np^5 (n ≧2)
9. Which species is diamagnetic?
(a) Cr^2+ (b)Zn (c) Mn (d) C
10. Which molecule would you expect to be a free radical?
(a) CO (b) CO_2 (c) N_2O (d) NO_2
11. Choose the molecule which is nonpolar.
(a) BCl_3 (b) NF_3 (c) ClF_3 (d) SO_3
12. Which molecule could have an expanded octet?
(a) H_2CO_3 (b) SF_6 (c) NO_2 (d) ClF_3
13. Which of the following substance would you expect to have little or no bandgap?
(a) Zn(s) (b) Si(s) (c)S(s) (d) Hg(l)
14. According to the critical point, which of the following substances cannot be liquefied at 25^。C?
(a) NH_3: 132^。C and 111 atm (b) N_2: -147^。C and 34 atm
(c) CO_2: 31^。C and 72.8 atm (d) cannot determine from critical point.
15. Under room temperature and pressure, which of the following ranking is correct?
(a) Boiling point: Cl_2 < Br_2 < I_2 (b) Vapor pressure: C_2H_5OC_2H_5 < CH_3OH < H_2O
(c) Viscosity: CH_2(OH)CH(OH)CH_2OH(l) < CH_3CH(OH)CH_2OH(l) < C_3H_5OH(l)
(d) Solubility in hexane: I_2(s) < NaCl(s)
II. Problems (70%)
1. Zinc metal reacts with hydrochloric acid according to this balanced equation. (4%)
Zn(s) + 2HCl(aq) →ZnCl_2(aq) + H_2(g)
When 0.122 g of Zn_(s) are combined with enough HCl to make 50.4 mL of solution in a coffee-cup calorimeter, all of the zinc reacts, raising the temperature of the solution from 22.0^。C. Find △H_rxn (in kJ/mol) for this reaction as written. (Use 1.0 g/mL for the density of the solution and 4.18 J/g-^。C as the specific heat capacity.) (4%)
2. Write an equation for the formation of each compound from its elements in their standard states: (4%)
(a) KBrO_3(s); (b) Fe(NO_3)_2(s).
3. For the balanced chemical reaction: CH_3OH(g) + 3/2O_2(g) → CO_2(g) + 2H_2O(g) (8%)
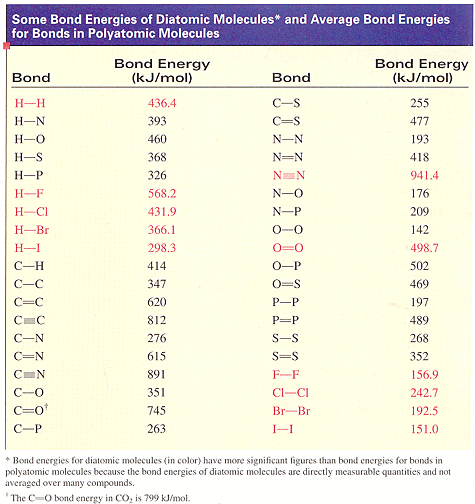
(a) Use bond energies to estimate the enthalpy change for the reaction.
(b) Calculate the enthalpy of reaction from the standard enthalpies of formation of the reactant and product molecules.
┌───────┬─────┬────┬────┐
│ Compound │CH_3OH(g) │CO_2(g) │H_2O(g) │
├───────┼─────┼────┼────┤
│△H_f(kJ/mol) │ -201 │ -394 │ -242 │
└───────┴─────┴────┴────┘
4. Writhe the electron configuration for Ge and identify the valence electrons and the core electrons. (4%)
5. The minimum energy required to cause the photoelectric effect in potassium meta is 3.69 ×10^-19 J. (8%)
(a) What is the threshold frequency of potassium to produce the photoelectric effect?
(b) What is the wave length in nm?
(c) Will photoelectrons be produced when visible light shines on the surface of potassium?
(d) If 400 nm radiation is shone on potassium, what is the kinetic energy of the ejected electrons?
6. Arrange the following elements in order of decreasing first ionization energy: S, Ca, F, Rb, Si. (2%)
7. Arrange the iso electronic series in order of decreasing radius: Cl^-, K^+, S^2-, Ca^2+. (2%)
8. Arrange these elements in order of decreasing electronegativity: P, Na, N, Al. (2%)
9. Consider the structure of the amino acid alanine shown as follows. (6%)
(a) Indicate the hybridization used by labeled atom (1), (2), and (3).
(b) Predict the approximate angles about (A), (B), and (C).
H H O
﹨ ∣ // (A) O = C —OH 考卷上原子是拉線標記
(1)N —(2)C —(3)C (B) N —C —CH_3 鍵角是標在圖中
/ ∣ ﹨ (C) H —N —H
H CH_3 OH
10. (a) Draw the resonance Lewis structures for the NO_2^- ion. (10%)
(b) Indicate the formal charges of each atom.
(c) Describe the hybrid orbitals used by the central atom.
(d) Predict the geometry of the NO_2^- ion.
(e) What is the bond order of N-O?
11. For C_2 molecule, use the molecular orbital model, (a) draw the energy level diagram, (b) write the electron configurations, (c) determine the bond order, and (d) indicate the magnetic property (that is diamagnetic or paramagnetic) for the molecule. (12%)
(e) Show the HOMO-LUMO energy gap in C_2 MO diagram.
(f) According to the bond order, compare the bond energy of C_2 and C_2^-.
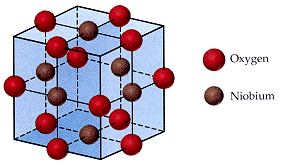
12. The niobium oxide crystallizes in the following cubic unit cell. (8%)
(a) Determine the number of atoms per unit cell for niobium(Nb) and oxygen atoms per unit cell.
(b) What is the emprical formula of niobium oxide?
(c) Identify the niobium oxide as molecular, ionic, atomic, or covalent network solid?
—————○—————
/| /| /│
∕| ∕│ ∕│
○ ┼———● ┼——— ○ │
/│ ○— —∕—●———/┼-○
∕│/| ∕ │ ∕│/│
┌-┼-┼-—○──┼— ┐ |/ │
│ ● │ │ │ │ ● │
│/│ ——┼——○——┼/┼—
|/ │/ │ / |/ │∕
○-┼-—-—●—/——— ○ |/
∣ ○———┼ ●——— ┼-○ ○Oxygen
│/ │/ │∕ ●Niobium
|/ |/ |/
└———— ○ ———— ┘
![]()
參考資料:
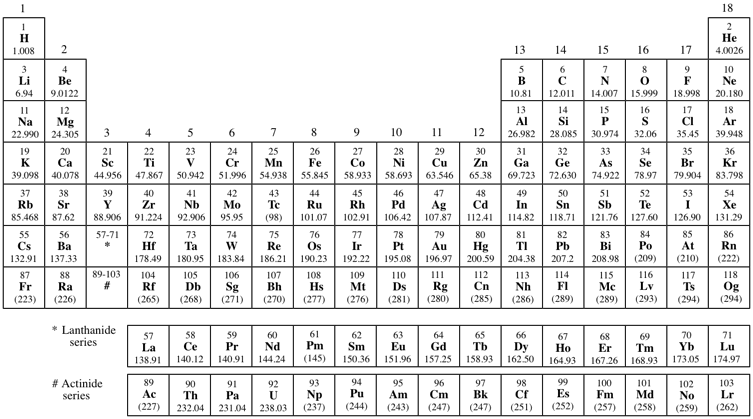
有原子序的週期表:
![]()
課本的鍵能表:
![]()
課程性質︰必修
課程教師︰佘瑞琳
開課學院:生命科學院
開課系所︰生科系/生技系
考試日期(年月日)︰2017.11.27
考試時限(分鐘):100+10(延長)
試題 :
C = 3.00 ×10^8 m/s; h = 6.626 ×10^-34 J-s; R_H = 1.096776 ×10^7 m^-1; 1 eV = 1.6 ×10^-19 J
Gas constant: R = 8.314 J/mol-K=0.082 L-atm/mol-K; 1 L-atm = 101.3 J
I. Multiple choices questions(30%)
1. When 1 mol of a fuel is burned at constant pressure. It release 3453 kJ of heat (q) and does 12kJ of work (w) on surroundings. Which one of the followings is true?(△E: internal energy)
(a) q = -3453 kJ (b) w = -12 kJ (c) △E_system = 0 kJ (d) △H = -3453
2. For the following types of electromagnetic radiation: γ-ray, ultraviolet (UV), visible, and infrared(IR), which of the following is correct?
(a) Highest energy: γ-ray (b) Longest wavelength: IR
(c) Greatest frequency: UV (d) Lowext energy: visible
3. What is the full range of possible values of l for n = 3?
(a) 0 (b) 0 and 1 (c) 0, 1, and 2 (d) 0, 1, 2, and 3
4. These sets of quantum numbers are each supposed to specify an orbital. Choose the one that is erroneous.
(a)(3, 0, 0) (b)(2, 1, -1) (c)(1, 0, 0) (d)(4, 1, -2)
5. Which electron transition emits light of the highest frequency in the hydrogen atom?
(a) n_i=4 →n_f=3 (b) n_i=5 →n_f=4 (c) n_i=2 →n_f=5 (d) n_i=4 →n_f=2
6. Which of the following orbital diagram is correct for the ground-state electron configurations?
(a) [He]↑↑ ↑ ↑ ↑ (b) [He]↑↓ ↓↑ ↓ ↓
﹉﹉2s ﹉﹉ ﹉﹉ ﹉﹉2p ﹉﹉2s ﹉﹉ ﹉﹉ ﹉﹉2p
(c) [He]↑ ↓↑ ↓↑ ↓↑ (d) [He]↑↓ ↑ ↓ ↑
﹉﹉2s ﹉﹉ ﹉﹉ ﹉﹉2p ﹉﹉2s ﹉﹉ ﹉﹉ ﹉﹉2p
7. How many orbitals have the quantum values of n = 3 and l = 2?
(a) 3 (b) 5 (c) 7 (d) 9 (e) 10 (f) 14
8. Choose the valence shell electron configurations of the alkali metals.
(a) ns^1 (b) ns^2 (c)ns^2np1 (d) ns^2np^5 (n ≧2)
9. Which species is diamagnetic?
(a) Cr^2+ (b)Zn (c) Mn (d) C
10. Which molecule would you expect to be a free radical?
(a) CO (b) CO_2 (c) N_2O (d) NO_2
11. Choose the molecule which is nonpolar.
(a) BCl_3 (b) NF_3 (c) ClF_3 (d) SO_3
12. Which molecule could have an expanded octet?
(a) H_2CO_3 (b) SF_6 (c) NO_2 (d) ClF_3
13. Which of the following substance would you expect to have little or no bandgap?
(a) Zn(s) (b) Si(s) (c)S(s) (d) Hg(l)
14. According to the critical point, which of the following substances cannot be liquefied at 25^。C?
(a) NH_3: 132^。C and 111 atm (b) N_2: -147^。C and 34 atm
(c) CO_2: 31^。C and 72.8 atm (d) cannot determine from critical point.
15. Under room temperature and pressure, which of the following ranking is correct?
(a) Boiling point: Cl_2 < Br_2 < I_2 (b) Vapor pressure: C_2H_5OC_2H_5 < CH_3OH < H_2O
(c) Viscosity: CH_2(OH)CH(OH)CH_2OH(l) < CH_3CH(OH)CH_2OH(l) < C_3H_5OH(l)
(d) Solubility in hexane: I_2(s) < NaCl(s)
II. Problems (70%)
1. Zinc metal reacts with hydrochloric acid according to this balanced equation. (4%)
Zn(s) + 2HCl(aq) →ZnCl_2(aq) + H_2(g)
When 0.122 g of Zn_(s) are combined with enough HCl to make 50.4 mL of solution in a coffee-cup calorimeter, all of the zinc reacts, raising the temperature of the solution from 22.0^。C. Find △H_rxn (in kJ/mol) for this reaction as written. (Use 1.0 g/mL for the density of the solution and 4.18 J/g-^。C as the specific heat capacity.) (4%)
2. Write an equation for the formation of each compound from its elements in their standard states: (4%)
(a) KBrO_3(s); (b) Fe(NO_3)_2(s).
3. For the balanced chemical reaction: CH_3OH(g) + 3/2O_2(g) → CO_2(g) + 2H_2O(g) (8%)
(a) Use bond energies to estimate the enthalpy change for the reaction.
(b) Calculate the enthalpy of reaction from the standard enthalpies of formation of the reactant and product molecules.
┌───────┬─────┬────┬────┐
│ Compound │CH_3OH(g) │CO_2(g) │H_2O(g) │
├───────┼─────┼────┼────┤
│△H_f(kJ/mol) │ -201 │ -394 │ -242 │
└───────┴─────┴────┴────┘
4. Writhe the electron configuration for Ge and identify the valence electrons and the core electrons. (4%)
5. The minimum energy required to cause the photoelectric effect in potassium meta is 3.69 ×10^-19 J. (8%)
(a) What is the threshold frequency of potassium to produce the photoelectric effect?
(b) What is the wave length in nm?
(c) Will photoelectrons be produced when visible light shines on the surface of potassium?
(d) If 400 nm radiation is shone on potassium, what is the kinetic energy of the ejected electrons?
6. Arrange the following elements in order of decreasing first ionization energy: S, Ca, F, Rb, Si. (2%)
7. Arrange the iso electronic series in order of decreasing radius: Cl^-, K^+, S^2-, Ca^2+. (2%)
8. Arrange these elements in order of decreasing electronegativity: P, Na, N, Al. (2%)
9. Consider the structure of the amino acid alanine shown as follows. (6%)
(a) Indicate the hybridization used by labeled atom (1), (2), and (3).
(b) Predict the approximate angles about (A), (B), and (C).
H H O
﹨ ∣ // (A) O = C —OH 考卷上原子是拉線標記
(1)N —(2)C —(3)C (B) N —C —CH_3 鍵角是標在圖中
/ ∣ ﹨ (C) H —N —H
H CH_3 OH
10. (a) Draw the resonance Lewis structures for the NO_2^- ion. (10%)
(b) Indicate the formal charges of each atom.
(c) Describe the hybrid orbitals used by the central atom.
(d) Predict the geometry of the NO_2^- ion.
(e) What is the bond order of N-O?
11. For C_2 molecule, use the molecular orbital model, (a) draw the energy level diagram, (b) write the electron configurations, (c) determine the bond order, and (d) indicate the magnetic property (that is diamagnetic or paramagnetic) for the molecule. (12%)
(e) Show the HOMO-LUMO energy gap in C_2 MO diagram.
(f) According to the bond order, compare the bond energy of C_2 and C_2^-.
12. The niobium oxide crystallizes in the following cubic unit cell. (8%)
(a) Determine the number of atoms per unit cell for niobium(Nb) and oxygen atoms per unit cell.
(b) What is the emprical formula of niobium oxide?
(c) Identify the niobium oxide as molecular, ionic, atomic, or covalent network solid?
—————○—————
/| /| /│
∕| ∕│ ∕│
○ ┼———● ┼——— ○ │
/│ ○— —∕—●———/┼-○
∕│/| ∕ │ ∕│/│
┌-┼-┼-—○──┼— ┐ |/ │
│ ● │ │ │ │ ● │
│/│ ——┼——○——┼/┼—
|/ │/ │ / |/ │∕
○-┼-—-—●—/——— ○ |/
∣ ○———┼ ●——— ┼-○ ○Oxygen
│/ │/ │∕ ●Niobium
|/ |/ |/
└———— ○ ———— ┘
參考資料:
有原子序的週期表:
課本的鍵能表:
繼續閱讀
[試題] 106上 陳逸昆 偏微分方程式一 期中考一BreathWay[試題] 106上 陳俊全 常微分方程導論 小考一t0444564[試題] 106上 陳俊全 常微分方程導論 期中考t0444564[試題] 106-1 林清富 近代物理 期中考Powerfulfox[公告] 目前公開要求考題不予公開的老師listcheetahfs[試題] 106-1 張志中 微積分甲上(物微) 期中考TunaVentw[試題] 106-1 汪信君 保險法 期中考ivstitia[試題] 106-1 蔡季廷 國際公法一 小考ETbaby0415[試題] 106-1 張佑宗 比較政府一 期中考ETbaby0415[試題] 106-1 沈俊嚴 實分析一 期中考craig100